Human Developmental Biology Resource

Our mission
The Human Developmental Biology Resource (HDBR) is a tissue bank comprising of prospective and archived collections of human embryonic and fetal material from 3 weeks of development onwards.
As a Human Tissue Authority licenced Research Tissue Bank with Research Ethics Committee approval, HDBR has permission to collect, store and distribute human fetal tissues up to 24 weeks gestation to registered users of the resource – meaning UK based researchers do not need to obtain their own ethics approval to use the material.
Following project review and approval by the HDBR, specific organs and anatomical structures can be dissected and processed following bespoke collections procedures and sent directly to researchers' laboratories. Alternatively, experimental analysis can be provided on your behalf by the experienced HDBR team through the In-House Gene Expression Service (IHGES).
In addition to providing access to material, HDBR also hosts the HDBR Atlas (www.hdbratlas.org). This website provides a portal to a plethora of human fetal gene expression data, and the website also serves as an educational a resource for anyone with an interest in early human development and anatomy.
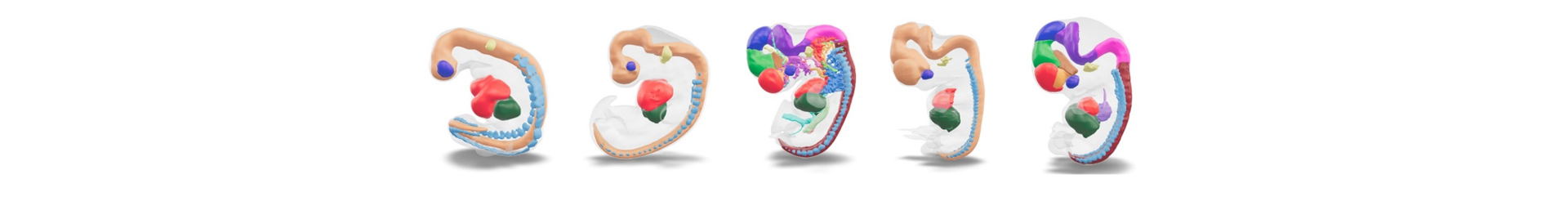
Available Services
Access to human developmental tissues (organ and stage specific tissues)
- Collected into media for cell culture
- Frozen, or processed into FFPE blocks
- Sectioned tissue on microscope slides
- DNA/RNA/cDNA preparations
- Bespoke dissections to cater for specific research needs
The HDBR In-House Gene Expression Service can provide experimental analysis on behalf of users including:
- In situ hybridisation - RNAScope, BaseScope
- Immunohistochemistry, chromogenic and fluorescent
- Spatial Transcriptomics (FFPE, Frozen)
- Nuclei isolations/single cell suspensions preparation for snRNA-seq
Workflow
Access to Tissue
- Project application submission to HDBR (http://hdbr.org/registration-forms)
- HDBR project review
- Formal project approval
- Collection of tissue (time dependent on sample availability)
- Release of tissue to researcher
- Retrospective formal ratification of project by Steering Committee
- Following publication, researchers must send all images produced from microscope slides to the HDBR to be included on the HDBR Atlas gene expression websites.
- At the end of the study, all remaining tissue must be returned to the HDBR or disposed following local and national guidelines.
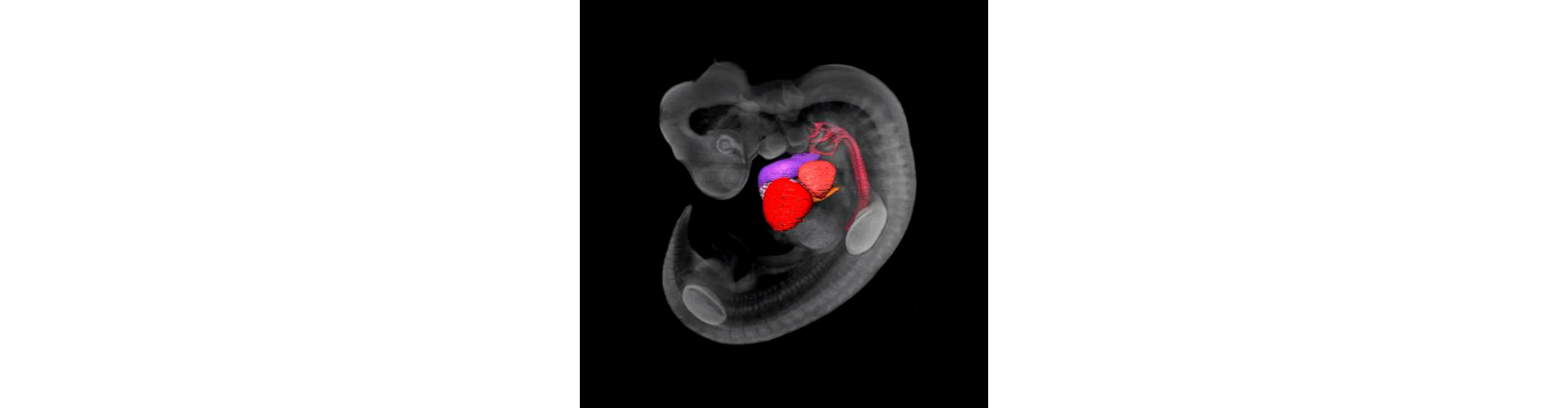
In-House Gene Expression Service (IHGES)
- Project application submission to HDBR (http://hdbr.org/registration-forms)
- HDBR project review and approval
- Preliminary meeting to discuss project specifics and goals with user
- Collection of tissue (if archived samples cannot be used)
- Experimental analysis conducted
- Results and data transferred to user
- Final report outlining observations
- Assistance with publication (e.g. production of high-resolution images)
- Once published, data is upload to HDBR Atlas website (www.hdbratlas.org)
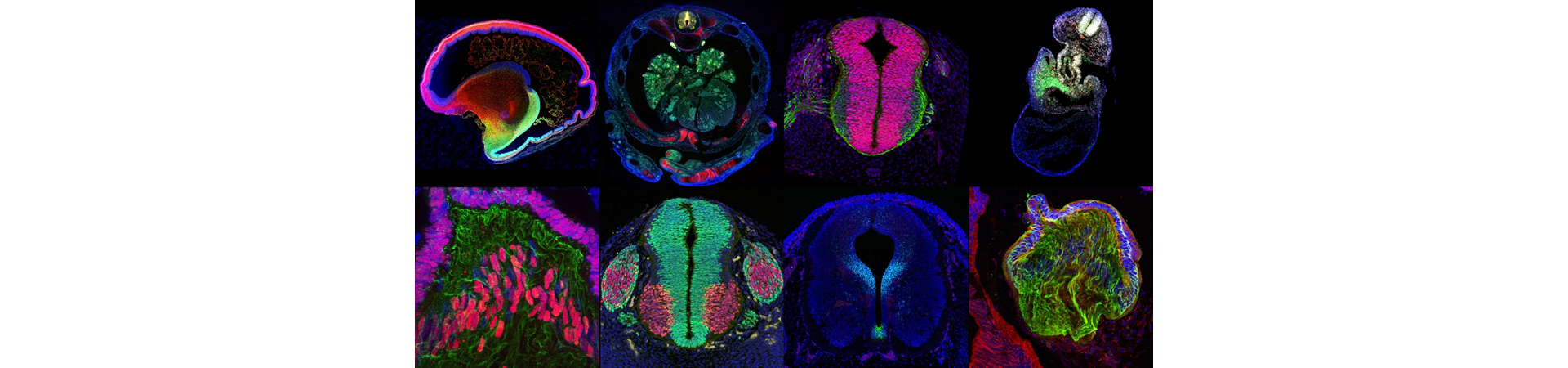
Applications
Human developmental tissue supplied by HDBR has successfully been used for many applications including:
- Bulk tissue and single cell/nuclei RNA and ATAC sequencing
- Cell and organoid culture
- In situ hybridisation and Immunohistochemistry
- Spatial Transcriptomics
- Histological analysis
- Quantitative trait loci (QTLs) and methylation quantitative trait loci (mQTLs) analysis
Service request
To access the material or services provided by the HDBR, an HDBR Project Registration form must be completed and returned via email to this address – hdbr@ncl.ac.uk. A Project registration form can be downloaded here.
For general enquiries and further information, please contact enquiries@hdbr.org.
FAQs
Which sections of the HDBR project registration form do I need to complete?
If only requesting experimental analysis through the IHGES, only sections 1 and 3 are required.
If requesting tissue to be released from the tissue bank for use in research within your own laboratory, all sections of the form must be completed.
Do I need an MTA?
If the research will take place entirely within Newcastle University, then an MTA is not required, and this section of the form should be kept blank. If material will also be sent to a collaborator on the project who is outside of Newcastle, then it will be necessary to establish an MTA with their institution. Additionally, if the collaborator is based overseas, ethical approval to use human fetal tissue in their research is required before any material can be released from the tissue bank.
How long will it take for my project to be registered with the HDBR?
Following receipt of a completed HDBR registration form, an internal review of the proposed project will take place and a response can be expected within two weeks. If it is deemed that the project clearly fits within the scope of the current HDBR ethical approval, the project can be registered immediately and material may be released with the tissue bank straight away. Formal ratification of the project by the HDBR’s Joint Steering Committee (JSC) will then be made at the next 6-montly meeting. Usually, the JSC do not request any amendments to the project registration form or the proposed study, but in the infrequent instances changes are requested by the committee, the researcher will need to action these changes before any further material can be provided.
Any contentious projects will be sent directly to the HDBR Project Review Committee to seek clarification as to whether the project may be supported, support with amendments to the study or registration form, or (extremely rarely) it falls outside of the scope of the HDBR ethical approval and in which case material can not be supplied for this study. A response to applicants of project proposals that have been sent to the Project Review Committee f can usually be expected within 3 months.
Where can I find a copy of the HDBR ethics approval?
The HDBR REC approval can be downloaded here - http://hdbr.org/ethical-approvals
Do I need to obtain ethical approval for use of the HDBR material?
Ethical permission for the collection of this material for research has been obtained from the Newcastle and North Tyneside 1 Research Ethics Committee. This approval extends to all UK based applicants using this material in their research, and will not usually need to obtain their own ethical approval prior to the release of tissue from the HDBR.
In an extremely limited number of cased, applicants will be required to obtain their own ethical approval from an NHS research ethics committee if their proposed project lies outside the activities for which the HDBR has pre-existing ethical approval, or if asked to do so by the HDBR’s oversight committee. Under such circumstances, no tissues can be distributed until documentary proof of ethical approval has been received.
What if I wish to publish my data?
The HDBR strongly encourages publications of research using our tissue bank. All publications arising from the use of material provided by the HDBR must acknowledge the contribution of the HDBR tissue bank within the manuscript. We would also encourage the publication of data within open access journals, a list of which can be found at http://www.doaj.org/.
Is the HDBR licenced to provide human genetic material?
Yes, the HDBR is licenced by the Human Tissue Authority (HTA) to provide human genetic material to users.
Staff
- Dr Steven Lisgo (Resource Manager)
- Lynne Overman (Experimental Scientific Officer)
- Jasmin Elana Turner (Experimental Scientific Officer)
- Tamilvendhan Dhanaseelan (Experimental Scientific Officer)
- Moira Crosier (Experimental Scientific Officer)
- Aragorn Jones (Experimental Scientific Officer)
- Janet Kerwin (HDBR Atlas editor)
- Jacqueline Dobor (Project Assistant)
