Newcastle Preclinical In Vivo Imaging Facility
Available Services
PIVI offers access and training to a range of clinically translatable in vivo imaging technologies, catering to a broad spectrum of research requirements. Through the Small Animal Models and Imaging (SAMI) special interest group, PIVI provides collaborative opportunities to access in vivo disease models that closely replicate patient conditions across various research areas.
Services
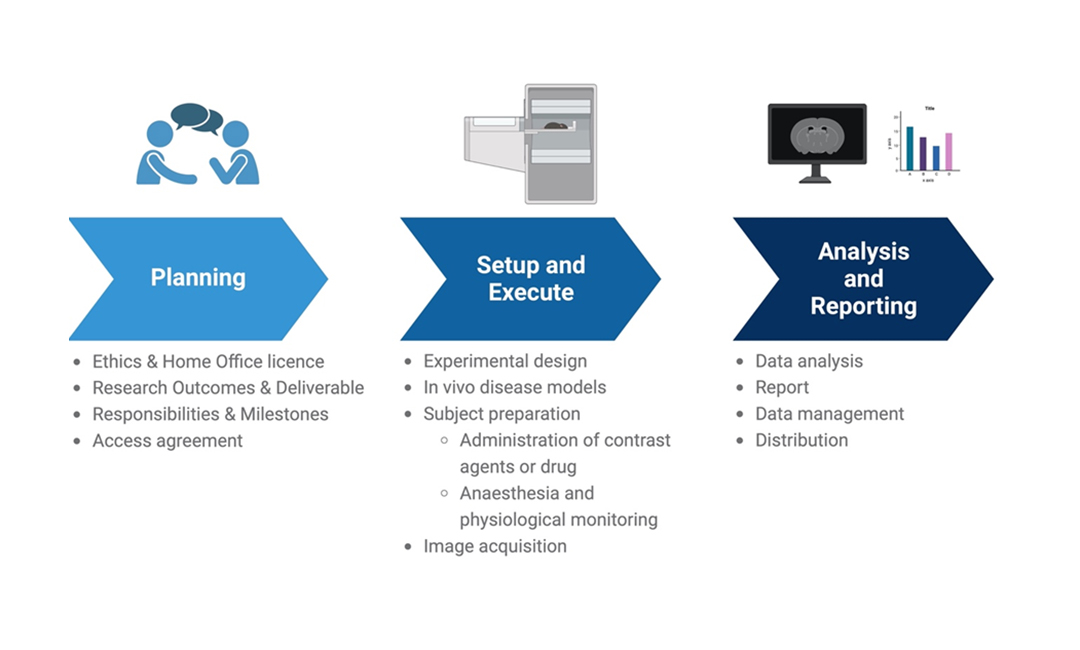
- Experimental design
- Image acquisition
- Image analysis
- Administration of drugs or contrast agents
- Routes of administration (IV, IP, subcut, oral etc.)
- Surgical procedures
- Intrahepatic injection
- Unilateral Ureter Obstruction (kidney fibrosis)
Technology
- In-vivo Imaging system (IVIS)
- Fluorescence
- Bioluminescence
- Micro Computerised Tomography (CT)
- Ultrasound-based Shear Wave Elastography (SWE)
Why is preclinical in vivo imaging important?
Preclinical in vivo imaging allows researchers to non-invasively visualize and analyse biological processes, study disease progression, test experimental treatments, and understand the efficacy and safety of interventions before advancing to human clinical trials.
How is preclinical imaging governed?
We strictly adhere to UK law in conducting all our animal research. Governed by the Animals (Scientific Procedures) Act 1986 (ASPA), this primary legislation delineates the legal and ethical obligations of researchers, establishments, and the Home Office. The Home Office, responsible for overseeing and granting licenses, has granted Newcastle University an establishment license. Our researchers hold project licenses, and our staff, possessing personal licenses, have undergone requisite training and competency assessments.
Research projects involving protected animals (as defined by ASPA) are reviewed by the Animal Welfare Ethical Review Body (AWERB).
Does preclinical imaging comply with the 3Rs principle (Replacement, Reduction, and Refinement)?
In vivo imaging enables researchers to gather comprehensive longitudinal data from individual animals over time, reducing the need for larger cohorts. This approach contributes to statistical rigor and the reduction of overall animal use in a study.
In vivo imaging techniques are often non-invasive or minimally invasive, reducing the stress and discomfort associated with traditional methods. This refinement enhances the welfare of animals by minimizing the invasiveness of procedures.
What types of research benefit from preclinical in vivo imaging?
Preclinical in vivo imaging is valuable in various research areas, including cancer, neurology, heart disease, immunology, and development of drugs and vaccines against infectious diseases like COVID-19. It helps researchers gain insights into diseases, assess treatment responses, and optimise experimental protocols.
What are the advantages of using preclinical in vivo imaging in research?
Advantages include non-invasiveness, real-time monitoring, the ability to study dynamic processes, and the acquisition of detailed anatomical and functional information, enhancing the understanding of complex biological systems.
How is image analysis handled in preclinical in vivo imaging?
Image analysis involves specialized software to process and interpret imaging data. Quantitative analysis tools help extract meaningful information, and researchers often use a combination of software for 2D/3D visualization and analysis.
Feedback
"We used the University CT scanning service to helps us improve our products. The scanning service was quick and of high quality. The team was easy to work with, knowledgeable and very helpful."
Martin Newman, Senior Quality Engineer, Parker Hannifin Manufacturing Ltd
"The technical team really engaged with us to understand the type of images that we needed and was responsive. The resolution of the images was very good and the details of the sequence in the DICOM header were very descriptive enabling accurate post-processing of the data."
Dr. Delphine Elbes, Engineering Manager, Orthoson
Publications
2024
- Pringle, T. A. et al. Synthesis and preclinical evaluation of a 89Zr-labelled human single chain antibody for non-invasive detection of hepatic myofibroblasts in acute liver injury. Scientific Reports 2024 14:1 14, 1–11 (2024).
2023
- Gristwood, K., Luli, S., Rankin, K. S. & Knight, J. C. Synthesis and In Vitro Evaluation of a HER2-Specific ImmunoSCIFI Probe. ACS Omega 8, 47905–47912 (2023).
- Bagga, M. et al. Assessing the potential application of bacteria-based self-healing cementitious materials for enhancing durability of wastewater treatment infrastructure. Cem Concr Compos 143, 105259 (2023).
- Zadran, B. et al. Impact of retrotransposon protein L1 ORF1p expression on oncogenic pathways in hepatocellular carcinoma: the role of cytoplasmic PIN1 upregulation. British Journal of Cancer 2023 128:7 128, 1236–1248 (2023).
- Gee, L. M. V. et al. Anti–Cholestatic Therapy with Obeticholic Acid Improves Short-Term Memory in Bile Duct–Ligated Mice. Am J Pathol 193, 11–26 (2023).
- Madgwick, S. et al. Claspin haploinsufficiency leads to defects in fertility, hyperplasia and an increased oncogenic potential. Biochemical Journal 479, 2115–2130 (2022).
2022
- Hunter, J. E. et al. Mutation of the RelA(p65) Thr505 phosphosite disrupts the DNA replication stress response leading to CHK1 inhibitor resistance. Biochemical Journal 479, 2087–2113 (2022).
- Hunter, J. E. et al. Up-regulation of the PI3K/AKT and RHO/RAC/PAK signalling pathways in CHK1 inhibitor resistant Eµ-Myc lymphoma cells. Biochemical Journal 479, 2131–2151 (2022).
- Hunter, J. E. et al. Regulation of CHK1 inhibitor resistance by a c-Rel and USP1 dependent pathway. Biochemical Journal 479, 2063–2086 (2022).
- Leslie, J. et al. CXCR2 inhibition enables NASH-HCC immunotherapy. Gut 71, 2093–2106 (2022).
- Pringle, T. A. et al. Synthesis and In Vivo Evaluation of a Site-specifically Labeled Radioimmunoconjugate for Dual-Modal (PET/NIRF) Imaging of MT1-MMP in Sarcomas. Bioconjug Chem 33, 1564–1573 (2022).
- Nadimi, S. et al. Observations of root growth in stratified soils at the microscopic scale: Insights from micro-computed tomography. EGUGA EGU22-12425 (2022) doi:10.5194/EGUSPHERE-EGU22-12425.
- Kemp, N., Angelidakis, V., Luli, S. & Nadimi, S. How Do Roots Interact with Layered Soils? Journal of Imaging 2022, Vol. 8, Page 5 8, 5 (2022).
- Gristwood, K., Luli, S., Rankin, K. S. & Knight, J. C. In situ excitation of BODIPY fluorophores by 89 Zr-generated Cerenkov luminescence. Chemical Communications 58, 11689–11692 (2022).
2021
- Kamala, O. et al. Homodimeric minimal factor H: in vivo tracking and extended dosing studies in factor H deficient mice. Front Immunol 12, 752916 (2021).
- Leslie, J. et al. Author Correction: c-Rel orchestrates energy-dependent epithelial and macrophage reprogramming in fibrosis. Nat Metab 3, 118–119 (2021).
- Lascurain, T., Angelidakis, V., Luli, S. & Nadimi, S. Imaging the root–rhizosphere interface using micro computed tomography: quantifying void ratio and root volume ratio profiles. in EPJ Web of Conferences vol. 249 11005 (EDP Sciences, 2021).
- Leslie, J., Robinson, S. M., Oakley, F. & Luli, S. Non-invasive synchronous monitoring of neutrophil migration using whole body near-infrared fluorescence-based imaging. Sci Rep 11, 1415 (2021).
2020
- Geh, D. et al. Hepatocellular carcinoma alters granulopoiesis to produce neutrophils with an immature phenotype. Immunology 21, 135–144 (2020).
- Leslie, J. et al. c-Rel orchestrates energy-dependent epithelial and macrophage reprogramming in fibrosis. Nat Metab 2, 1350–1367 (2020).
- Tual-Chalot, S. et al. Loss of endothelial endoglin in adult life leads to peripheral arteriovenous shunting and high output heart failure. In cardiovascular drugs and therapy vol. 34 277 (springer van godewijckstraat 30, 3311 gz dordrecht, netherlands, 2020).
- Leslie, J. et al. FPR-1 is an important regulator of neutrophil recruitment and a tissue-specific driver of pulmonary fibrosis. JCI Insight 5, (2020).
2019
- Moran-Salvador, E. et al. Fibrogenic activity of MECP2 is regulated by phosphorylation in hepatic stellate cells. Gastroenterology 157, 1398–1412 (2019).
- Sirbu, D., Luli, S., Leslie, J., Oakley, F. & Benniston, A. C. Enhanced in vivo Optical Imaging of the Inflammatory Response to Acute Liver Injury in C57BL/6 Mice Using a Highly Bright Near‐Infrared BODIPY Dye. ChemMedChem 14, 995–999 (2019).
- Concetti, J. et al. NFKB1 acts as a hepatocyte-specific tumour suppressor. in FEBS OPEN BIO vol. 9 58 (WILEY 111 RIVER ST, HOBOKEN 07030-5774, NJ USA, 2019).
- Gee, L. et al. High throughput RNA sequencing unravels pathways associated with cognitive deficit in primary billiary cholangitis in Journal Of Hepatology vol. 70 e164–e165 (elsevier science bv PO Box 211, 1000 AE Amsterdam, Netherlands, 2019).
2018
- Isa, A. et al. Identification of glycolytic pathway as RUNX1/ETO-dependent for propagation and survival. Klin Padiatr 230, 3 (2018).
- Hunter, J. E. et al. Regulation of checkpoint kinase signalling and tumorigenesis by the NF-κB regulated gene, CLSPN. BioRxiv 358291 (2018).
2016
- Luli, S. et al. A new fluorescence-based optical imaging method to non-invasively monitor hepatic myofibroblasts in vivo. J Hepatol 65, 75–83 (2016).
2015
- Wilson, C. L. et al. NFκB1 is a suppressor of neutrophil-driven hepatocellular carcinoma. Nat Commun 6, 6818 (2015).
2013
- Moles, A. et al. Inhibition of RelA‐Ser536 phosphorylation by a competing peptide reduces mouse liver fibrosis without blocking the innate immune response. Hepatology 57, 817–828 (2013).
Leaf Gold Award
Following an audit we have been awarded a GOLD award for lab sustainability.
The LEAF programme, short for the Laboratory Efficiency Assessment Framework, is a self-assessment tool targeted at research, teaching and medical laboratories.
LEAF contains actions which lab users can take to save plastics, water, energy and other resources. By taking part in the programme, laboratories reduce their carbon emissions and create an environment that supports research quality.